NMN (β-Nicotinamide Mononucleotide)
Introduce

NMN is the direct precursor of nicotinamide adenine dinucleotide (NAD+) and is considered a key component to increase NAD+ levels in cells.
NAD+ is essential enzyme required for life and cellular functions. In animal studies, raising NAD+ levels in the body have shown promising results in research fields like metabolic and age-related disease and has even shown some anti-aging properties*. Age-related illnesses such as diabetes, cardiovascular diseases, neurodegeneration and general decreases in the immune system*.
By middle age, our NAD+ levels have plummeted to half that of our youth. Numerous studies have demonstrated that boosting NAD+ levels increases insulin sensitivity, reverses mitochondrial dysfunction, and extends lifespan. NAD+ levels can be increased by activating enzymes that stimulate synthesis of NAD+, by inhibiting an enzyme (CD38) that degrades NAD+, and by supplementing with NAD precursors, nicotinamide mononucleotide (NMN).
- Helping to keep healthy aging and NAD+ level
- Supported by multi-medical researches*
- *This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. The information provided on this site is intended for your general knowledge only and is not a substitute for professional medical advice or treatment for specific medical conditions.
Research
Here, you can read some of important published clinical research papers about NMN, which support its efficacy on improving blood Nicotinamide Adenine Dinucleotide Levels, rejuvenating brain cells and Published peer reviewed studies can be accessed through PubMed at www.ncbi.nlm.nih.gov/pubmed. For more information, please contact This email address is being protected from spambots. You need JavaScript enabled to view it..
Clinical Study Indicates that NMN Efficaciously Increases Blood NAD+ in Humans

Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects
Abstract: Nicotinamide mononucleotide (NNM) is an orally bioavailable NADC precursor that has demonstrated beneficial effects against aging and aging-associated diseases in animal models. NMN is ultimately converted to NADC, a redox cofactor that mediates many metabolic enzymes. NADC also serves as the substrate for poly (ADP-ribose) polymerase (PARP) and sirtuins, and regulates various biological processes, such as metabolism, DNA repair, gene expression, and stress responses. Previous mouse models showed that NMN administration can increase NADC in various organs and ameliorate aging related diseases, such as obesity, diabetes, heart failure, stroke, kidney failure, and Alzheimer’s disease through NADC-mediated pathways. However, evidence of its effect on humans is still scarce. In this study, we conducted a placebo-controlled, randomized, double blind, parallel-group trial to investigate the safety of orally administered NMN and its efficacy to increase NADC levels in thirty healthy subjects. Healthy volunteers received 250 mg/day of NMN (n = 15) or placebo (n = 15) for 12 weeks, and physiological and laboratory tests were performed during this period. In addition, NADC and its related metabolites in whole blood were examined. Oral supplementation of NMN for 12 weeks caused no abnormalities in physiological and laboratory tests, and no obvious adverse effects were observed. NADC levels in whole blood were significantly increased after NMN administration. We also observed the significant rise in nicotinic acid mononucleotide (NAMN) levels, but not in NMN. We also found that the increased amount of NADC was strongly correlated with pulse rate before the administration of NMN. These results suggest that oral administration of NMN is a safe and practical strategy to boost NADC levels in humans.
Source: Front. Nutr. 9:868640. doi: 10.3389/fnut.2022.868640
NMN Increases Telomere Length in Middle-Aged Adults

The Impacts of Short-Term NMN Supplementation on Serum Metabolism, Fecal Microbiota, and Telomere Length in Pre-Aging Phase
Abstract: Aging is a natural process with concomitant changes in the gut microbiota and associate metabolomes. Beta-nicotinamide mononucleotide, an important NAD+ intermediate, has drawn increasing attention to retard the aging process. We probed the changes in the fecal microbiota and metabolomes of pre-aging male mice (C57BL/6, age: 16 months) following the oral short-term administration of nicotinamide mononucleotide (NMN). Considering the telomere length as a molecular gauge for aging, we measured this in the peripheral blood mononuclear cells (PBMC) of pre-aging mice and human volunteers (age: 45-60 years old). Notably, the NMN administration did not influence the body weight and feed intake significantly during the 40 days in pre-aging mice. Metabolomics suggested 266 upregulated and 58 downregulated serum metabolites. We identified 34 potential biomarkers linked with the nicotinamide, purine, and proline metabolism pathways. Nicotinamide mononucleotide significantly reduced the fecal bacterial diversity (p < 0.05) with the increased abundance of Helicobacter, Mucispirillum, and Faecalibacterium, and lowered Akkermansia abundance associated with nicotinamide metabolism. We propose that this reshaped microbiota considerably lowered the predicated functions of aging with improved immune and cofactors/vitamin metabolism. Most notably, the telomere length of PBMC was significantly elongated in the NMN-administered mice and humans. Taken together, these findings suggest that oral NMN supplementation in the pre-aging stage might be an effective strategy to retard aging. We recommend further studies to unravel the underlying molecular mechanisms and comprehensive clinical trials to validate the effects of NMN on aging.
Source: Front Nutr. 2021 Nov 29;8:756243. doi: 10.3389
Researchers Show NMN Rejuvenates Brain Cells In Aged Mice

Restoring nuclear entry of Sirtuin 2 in oligodendrocyte progenitor cells promotes remyelination during ageing
Abstract: The age-dependent decline in remyelination potential of the central nervous system during ageing is associated with a declined differentiation capacity of oligodendrocyte progenitor cells (OPCs). The molecular players that can enhance OPC differentiation or rejuvenate OPCs are unclear. Here we show that, in mouse OPCs, nuclear entry of SIRT2 is impaired and NAD+ levels are reduced during ageing. When we supplement β-nicotinamide mononucleotide (β-NMN), an NAD+ precursor, nuclear entry of SIRT2 in OPCs, OPC differentiation, and remyelination were rescued in aged animals. We show that the effects on myelination are mediated via the NAD+-SIRT2-H3K18Ac-ID4 axis, and SIRT2 is required for rejuvenating OPCs. Our results show that SIRT2 and NAD+ levels rescue the aged OPC differentiation potential to levels comparable to young age, providing potential targets to enhance remyelination during ageing.
Source: Nat Commun. 2022 Mar 9;13(1):1225. doi: 10.1038/s41467-022-28844-1. PMID: 35264567
Study Shows NMN May Treat Rare Transmissible Neurodegenerative Disorder

PINK1-parkin-mediated neuronal mitophagy deficiency in prion disease
Abstract: A persistent accumulation of damaged mitochondria is part of prion disease pathogenesis. Normally, damaged mitochondria are cleared via a major pathway that involves the E3 ubiquitin ligase parkin and PTEN-induced kinase 1 (PINK1) that together initiate mitophagy, recognize and eliminate damaged mitochondria. However, the precise mechanisms underlying mitophagy in prion disease remain largely unknown. Using prion disease cell models, we observed PINK1-parkin-mediated mitophagy deficiency in which parkin depletion aggravated blocked mitochondrial colocalization with LC3-II-labeled autophagosomes, and significantly increased mitochondrial protein levels, which led to inhibited mitophagy. Parkin overexpression directly induced LC3-II colocalization with mitochondria and alleviated defective mitophagy. Moreover, parkin-mediated mitophagy was dependent on PINK1, since PINK1 depletion blocked mitochondrial Parkin recruitment and reduced optineurin and LC3-II proteins levels, thus inhibiting mitophagy. PINK1 overexpression induced parkin recruitment to the mitochondria, which then stimulated mitophagy. In addition, overexpressed parkin and PINK1 also protected neurons from apoptosis. Furthermore, we found that supplementation with two mitophagy-inducing agents, nicotinamide mononucleotide (NMN) and urolithin A (UA), significantly stimulated PINK1-parkin-mediated mitophagy. However, compared with NMN, UA could not alleviate prion-induced mitochondrial fragmentation and dysfunction, and neuronal apoptosis. These findings show that PINK1-parkin-mediated mitophagy defects lead to an accumulation of damaged mitochondria, thus suggesting that interventions that stimulate mitophagy may be potential therapeutic targets for prion diseases.
Source: Cell Death Dis. 2022 Feb 18;13(2):162. doi: 10.1038/s41419-022-04613-2. PMID: 35184140.
NMN Rejuvenates Bone Tissue Stem Cells
NAP1L2 drives mesenchymal stem cell senescence and suppresses osteogenic differentiation
Abstract: Senescence of bone marrow mesenchymal stem cells (BMSCs) impairs stemness and osteogenic differentiation, but the key regulators for senescence and the related osteogenesis are not well defined. Herein, we screened the gene expression profiles of human BMSCs from young and old donors and identified that elevation of the nucleosome assembly protein 1-like 2 (NAP1L2) expression was correlated with BMSC senescence and impaired osteogenesis. Elevated NAP1L2 expression was observed in replicative cell senescence and induced cell senescence in vitro, and in age-related senescent human and mouse BMSCs in vivo, concomitant with significantly augmented chromatin accessibility detected by ATAC-seq. Loss- and gain-of-functions of NAP1L2 affected activation of NF-κB pathway, status of histone 3 lysine 14 acetylation (H3K14ac), and chromatin accessibility on osteogenic genes in BMSCs. Mechanistic studies revealed that NAP1L2, a histone chaperone, recruited SIRT1 to deacetylate H3K14ac on promoters of osteogenic genes such as Runx2, Sp7, and Bglap and suppressed the osteogenic differentiation of BMSCs. Importantly, molecular docking analysis showed a possible bond between NAP1L2 and an anti-aging reagent, the nicotinamide mononucleotide (NMN), and indeed, administration of NMN alleviated senescent phenotypes of BMSCs. In vivo and clinical evidence from aging mice and patients with senile osteoporosis also confirmed that elevation of NAP1L2 expression was associated with suppressed osteoblastogenesis. Taken together, our findings suggest that NAP1L2 is a regulator of both BMSC cell senescence and osteogenic differentiation, and provide a new theoretical basis for aging-related disease.
Source: Aging Cell. 2022 Jan 15:e13551. doi: 10.1111/acel.13551. Epub ahead of print. PMID: 35032339.
Study Shows NMN Stops Blood Vessel Aging in Mice

CD38 deficiency alleviates Ang II-induced vascular remodeling by inhibiting small extracellular vesicle-mediated vascular smooth muscle cell senescence in mice
Abstract: CD38 is the main enzyme for nicotinamide adenine dinucleotide (NAD) degradation in mammalian cells. Decreased NAD levels are closely related to metabolic syndromes and aging-related diseases. Our study showed that CD38 deficiency significantly alleviated angiotensin II (Ang II)-induced vascular remodeling in mice, as shown by decreased blood pressures; reduced vascular media thickness, media-to-lumen ratio, and collagen deposition; and restored elastin expression. However, our bone marrow transplantation assay showed that CD38 deficiency in lymphocytes led to lack of protection against Ang II-induced vascular remodeling, suggesting that the effects of CD38 on Ang II-induced vascular remodeling might rely primarily on vascular smooth muscle cells (VSMCs), not lymphocytes. In addition, we observed that CD38 deficiency or NAD supplementation remarkably mitigated Ang II-induced vascular senescence by suppressing the biogenesis, secretion, and internalization of senescenceassociated small extracellular vesicles (SA-sEVs), which facilitated the senescence of neighboring non-damaged VSMCs. Furthermore, we found that the protective effects of CD38 deficiency on VSMC senescence were related to restoration of lysosome dysfunction, particularly with respect to the maintenance of sirtuin-mediated mitochondrial homeostasis and activation of the mitochondria–lysosomal axis in VSMCs. In conclusion, our findings demonstrated that CD38 and its associated intracellular NAD decline are critical for Ang II-induced VSMC senescence and vascular remodeling.
Source: Signal Transduct Target Ther. 2021 Jun 11;6(1):223. doi: 10.1038/s41392-021-00625-0. PMID: 34112762.
Aged Lung Cells Protected from Functional Decline with NMN
Nicotinamide mononucleotide ameliorates senescence in alveolar epithelial cells.
Abstract: Alveolar epithelial cells (ACEs) gradually senescent as aging, which is one of the main causes of respiratory defense and function decline. Investigating the mechanisms of ACE senescence is important for understanding how the human respiratory system works. NAD+ is reported to reduce during the aging process. Supplementing NAD+ intermediates can activate sirtuin deacylases (SIRT1– SIRT7), which regulates the benefits of exercise and dietary restriction, reduce the level of intracellular oxidative stress, and improve mitochondrial function, thereby reversing cell senescence. We showed that nicotinamide mononucleotide (NMN) could effectively mitigate age-associated physiological decline in the lung of 8–10 months old C57BL/6 mice and bleomycin-induced pulmonary fibrosis in young mice of 6–8 weeks. Besides, the treatment of primary ACEs with NMN can markedly ameliorate cell senescence phenotype in vitro. These findings to improve the respiratory system function and reduce the incidence and mortality from respiratory diseases in the elderly are of great significance.
Source: MedComm. 2021;2:279–287. https://doi.org/10.1002/mco2.62
NMN Improves Mouse Heart Dysfunction Caused by Scarring

Nicotinamide mononucleotide attenuates isoproterenol-induced cardiac fibrosis by regulating oxidative stress and Smad3 acetylation.
Abstract: Aims Cardiac fibrosis is a pathological hallmark of progressive heart diseases currently lacking effective treatment. Nicotinamide mononucleotide (NMN), a member of the vitamin B3 family, is a defined biosynthetic precursor of nicotinamide adenine dinucleotide (NAD⁺). Its beneficial effects on cardiac diseases are known, but its effects on cardiac fibrosis and the underlying mechanism remain unclear. We aimed to elucidate the protective effect of NMN against cardiac fibrosis and its underlying mechanisms of action. Materials and methods Cardiac fibrosis was induced by isoproterenol (ISO) in mice. NMN was administered by intraperitoneal injection. In vitro, cardiac fibroblasts (CFs) were stimulated by transforming growth factor-beta (TGF-β) with or without NMN and sirtinol, a SIRT1 inhibitor. Levels of cardiac fibrosis, NAD⁺/SIRT1 alteration, oxidative stress, and Smad3 acetylation were evaluated by real-time polymerase chain reaction, western blots, immunohistochemistry staining, immunoprecipitation, and assay kits. Key findings ISO treatment induced cardiac dysfunction, fibrosis, and hypertrophy in vivo, whereas NMN alleviated these changes. Additionally, NMN suppressed CFs activation stimulated by TGF-β in vitro. Mechanistically, NMN restored the NAD⁺/SIRT1 axis and inhibited the oxidative stress and Smad3 acetylation induced by ISO or TGF-β. However, the protective effects of NMN were partly antagonized by sirtinol in vitro. Significance NMN could attenuate cardiac fibrosis in vivo and fibroblast activation in vitro by suppressing oxidative stress and Smad3 acetylation in a NAD⁺/SIRT1-dependent manner.
Source: Life Sci. 2021 Mar 3;274:119299. doi: 10.1016/j.lfs.2021.119299. Epub ahead of print. PMID: 33675899.
FAQs
NMN stands for nicotinamide mononucleotide, a molecule naturally occurring in all life forms. At the molecular level, it is a ribo-nucleotide, which is a basic structural unit of the nucleic acid RNA. Structurally, the molecule is composed of a nicotinamide group, a ribose and a phosphate group. NMN is the direct precursor of the essential molecule nicotinamide adenine dinucleotide (NAD+) and is considered a key component to increase NAD+ levels in cells. Source: www.nmn.com
NMN has been shown to boost levels of an essential molecule with crucial roles in over 400 metabolic reactions called nicotinamide adenine dinucleotide (NAD+). Falling NAD+ levels with age have been linked to age-related conditions in animals like mice and rats and even humans, but increasing NAD+ with NMN improves these ailments.* Source: www.nmn.com
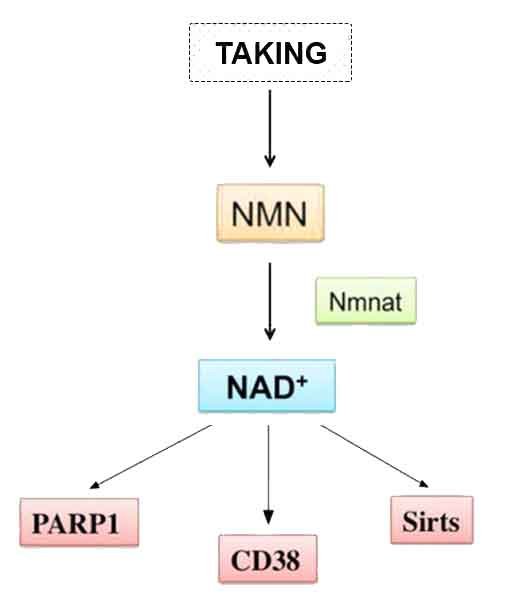
NMN has been shown to boost levels of nicotinamide adenine dinucleotide (NAD+). NAD+ is the most abundant molecule in the body besides water, and without it, an organism would die. NAD+ is used by many proteins throughout the body, such as the sirtuins, which repair damaged DNA. It is also important for mitochondria, which are the powerhouses of the cell and generate the chemical energy that our bodies use.
By middle age, our NAD+ levels have plummeted to half that of our youth. Age-related NAD+ level reductions have been linked to a plethora of age-related human diseases, and some studies provide evidence that increasing NAD+ combats these conditions. NMN studies from institutes like Harvard University and Washington University have shown that supplementing with the molecule or enhancing NMN synthesis promotes longevity and health during aging in rodents. Sinclair and colleagues found that when aged mice drank NMN-infused water, their running endurance almost doubled. Further studies have demonstrated that injecting mice with NMN preserves cognition during aging. Moreover, a study from Imai and colleagues indicates that an upsurge in NMN synthesis more than doubles the remaining lifespan of mice.* Source: www.nmn.com
Taking NMN may provide a promising means to combat age-related diseases and ailments. Below is a summary of major studies elucidating the potential benefits of NMN. Source: www.nmn.com
Scientists believe that the decline of NAD+ underpins many of the health-related problems that we face as we age. If we could stem this loss, the thinking goes, we might both live longer while remaining healthier.
One way that we might sustain healthy levels of NAD+ is by supplementing our bodies with its precursor, nicotinamide mononucleotide (NMN). All cellular compounds are made in a way analogous to a factory assembly line, where each component is the precursor for the next one. To produce more NAD+, then, one needs more precursors like NMN.
Promotes Vascular Health and Blood Flow
Studies in mice have shown that NMN protects against numerous aging-related declines in vascular health, such as the stiffening of blood vessels, oxidative stress, our cells’ ability to keep dividing, and even changes in how active our genes are, what scientists call gene expression.
Improves Muscle Endurance and Strength
We rely on our skeletal muscles for movement, stability, and strength. To remain strong and in good condition, these muscles must consume significant amounts of key energy molecules, like glucose and fatty acids. Because NAD+ is required to metabolize these molecules, our muscles need a steady supply of its building blocks, such as NMN.
Studies have shown that mice fed NMN for extended periods of time had better energy metabolism with no obvious side effects. The health of our muscles grows ever more important as we age and our own supply of NAD+ declines.
Protects Against Heart Disease
While your skeletal muscles get to take breaks, your heart does not get to rest. This blood-pumping organ can’t even slow its pace much without causing serious problems. The heart’s energy requirement, therefore, is tremendous. And to keep it ticking, it needs to make all the NAD+ it can. This is why heart cells need a steady supply of NMN.
Enhances Maintenance of DNA Repair
The NAD+ made from NMN activates a group of proteins called sirtuins — sometimes thought of as the guardians of our healthspan. Sirtuins play a key role in maintaining DNA integrity, which is constantly being bombarded by DNA altering substances (mutagens) like UV radiation. Also, each time our cells divide, the DNA at the very ends of our chromosomes grows a tiny bit shorter. At a certain point, this begins to damage our genes. Sirtuins slow this process by stabilizing those end bits, known scientifically as telomeres.
Since sirtuins rely upon NAD+ to function, there has been an effort to enhance sirtuin activity through NAD+ boosting methods. Along these lines, several recent studies have demonstrated that feeding mice NMN activated sirtuins and led to more stable telomeres.
Increases Mitochondrial Function
Simply put, we couldn’t live without our mitochondria. These unique cellular structures, which even have their own DNA, are known as the powerhouses of the cell. Mitochondria are critical for metabolism; that is, they convert molecules from the food we eat into the energy that our cells use.
At the very core of metabolism is NAD+. Without NAD+, mitochondria cannot metabolize and cells will be left without energy, resulting in their death. In fact, mitochondrial anomalies caused by the loss of NAD+ may even impact neurological disorders, such as Alzheimer’s. Studies done in mice have shown that NMN supplementation has rescued some mitochondrial dysfunctions.
Lowers Risk of Obesity
Obesity is linked to a wide array of unhealthy conditions and can be very challenging to treat. There is no easy remedy for obesity and related conditions such as diabetes and metabolic syndrome. While lifestyle adjustments like consistent exercise and a healthy diet are of paramount importance, every little bit helps.
In mouse studies, NMN displays an effect that mimics aspects of calorie restriction, which has been shown to provide numerous benefits to aging and health. However, calorie restriction is a difficult regime to maintain over a long period of time. Mimicking its benefits without adhering to such an extreme diet would be undeniably beneficial.
Source: www.nmn.com
An international team of researchers ran the first human clinical study for NMN in Japan to investigate the safety of the molecule. Although the size of the Phase 1 clinical trial was small, the study showed that dosages up to 500 mg of orally administered NMN are safe in humans, implicating a potential therapeutic strategy. The results appeared in the journal Endocrine, November 2019.
NMN’s safety as a dietary supplement has been proven in a number of FDA-approved clinical trials.
Other clinical trials registered with the World Health Organization (WHO) are also examining the safety and efficacy of NMN. In the US, researchers at Washington University School of Medicine are running a clinical trial to test NMN’s effect on cardiovascular and metabolic health with a daily dosage of 250 mg. Another clinical study at Brigham and Women’s Hospital in Boston is also testing the supplement’s effects on the body and if there are any side effects.
Currently, no side effects of nicotinamide mononucleotide have been documented in humans. Researchers have conducted the majority of studies on NMN in rodents, which revealed positive effects on metabolism, brain function, liver, skin, muscle, bone structure, heart health, reproduction, immunity, and lifespan. Long-term mice study also showed no toxicity, serious side effects, or increased mortality rate throughout the 12 month intervention period.
Source: www.nmn.com
Taking the human and animal studies of efficacy and safety into consideration and calculating optimal NMN doses, the recommended dose that adults between 30 and 60 years old take is 500 mg per day. People over age 65 can safely take 750 mg per day to maximize NMN’s benefits. Source: www.nmn.com
- *This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. The information provided on this site is intended for your general knowledge only and is not a substitute for professional medical advice or treatment for specific medical conditions.




