记忆强化胶囊 (CognitiPlus)
产品简介
记忆强化胶囊是大脑健康的特殊补品。 其主要活性成分,SynapsaTM 是Bacopa monieri 的专利标准化提取物,它具有30多年的临床研究历史,已被证明有助于在认知要求苛刻的环境(如考试)中帮助改善视觉处理能力,提高学习率,增加工作记忆和信息保持能力,强化思维能力。SynapsaTM 可以帮助降低健忘率并提高同时处理多重任务的准确性。*
研究表明SynapsaTM 在改善记忆方面表现出两种不同类型的益处。远期效果:每日服用,长期使用可以增强学习和记忆;近期效果:短期使用可以改善认知要求苛刻的环境(如考试)中的思维能力。*
Bacopa monnieri 是传统阿育吠陀医学中的一种常用草药,它的主要机制是促进神经元通讯。 Bacopa monnieri 促进神经末梢(树突)的生长,从而增加大脑与身体交流的速度。
Bacopa monnieri 也被归类为适应原。 适应原是一种天然物质,被用于封闭压力化学物质,从而对您的生理产生负调节效应。它通过特异性的减少生物标志物HSP70的含量来实现这一点。这最终会减少您和您的大脑所感受到的压力,让您更好地适应环境中潜在的压力因素。
更多专业研究请参阅《临床研究》
本产品诚招经销商和批发商
- * 本声明未经美国食品药品管理局评估。 本产品不用于诊断,治疗或预防任何疾病。 本网站提供的信息仅供您一般参考,不能代替专业医疗建议或针对特定疾病的治疗。
临床研究
Here, you can read some of important published clinical research papers about SynapsaTM , which support brain health. Published peer reviewed studies can be accessed through PubMed at www.ncbi.nlm.nih.gov/pubmed. For more information, please contact Renylin Bio-Health Inc.
Significantly improves speed of visual information processing, learning rate, memory consolidation

The chronic effects of an extract of Bacopa monniera on cognitive function in healthy human subjects
Abstract.
Rationale: Extracts of Bacopa monniera have been reported to exert cognitive enhancing effects in animals. However, the effects on human cognition are inconclusive. Objective: The current study examined the chronic effects of an extract of B. monniera (Keenmind) on cognitive function in healthy human subjects. Methods: The study was a double-blind placebo-controlled independent-group design in which subjects were randomly allocated to one of two treatment conditions, B. monniera (300 mg) or placebo. Neuropsychological testing was conducted pre-(baseline) and at 5 and 12 weeks postdrug administration. Results: B. monniera significantly improved speed of visual information processing measured by the IT task, learning rate and memory consolidation measured by the AVLT (P<0.05), and state anxiety (P<0.001) compared to placebo, with maximal effects evident after 12 weeks. Conclusions: These finding suggests that B. monniera may improve higher order cognitive processes that are critically dependent on the input of information from our environment such as learning and memory.
Stough et al. The chronic effects of an extract of Bacopa monniera on cognitive function in healthy human subjects, Psychopharmacology, 2001; 156:481-484.
A significant decrease in the rate of forgetting newly acquired information in individuals taking Synapsa.

Chronic effects of Brahmi on human memory
A study is reported on the effects of Brahmi (Bacopa monniera) on human memory. Seventy-six adults aged between 40 and 65 years took part in a double-blind randomized, placebo control study in which various memory functions were tested and levels of anxiety measured. There were three testing sessions: one prior to the trial, one after three months on the trial, and one six weeks after the completion of the trial. The results show a significant effect of the Brahmi on a test for the retention of new information. Follow-up tests showed that the rate of learning was unaffected, suggesting that Brahmi decreases the rate of forgetting of newly acquired information. Tasks assessing attention, verbal and visual short-term memory and the retrieval of pre-experimental knowledge were unaffected. Questionnaire measures of everyday memory function and anxiety levels were also unaffected.
Source:Roodenrys et al. Neuropsychopharmacology, 2002; 27:279-281.
Significant improvement of mental control, logical memory and paired associated learning
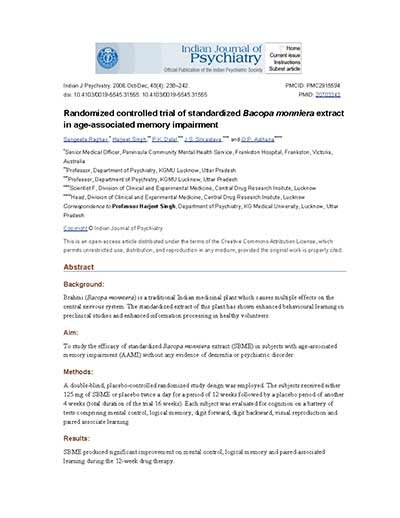
Randomized controlled trial of standardized Bacopa monniera extract in age-associated memory impairment
Abstract
Background: Brahmi (Bacopa monniera) is a traditional Indian medicinal plant which causes multiple effects on the central nervous system. The standardized extract of this plant has shown enhanced behavioural learning in preclinical studies and enhanced information processing in healthy volunteers.
Aim: To study the efficacy of standardized Bacopa monniera extract (SBME) in subjects with age-associated memory impairment (AAMI) without any evidence of dementia or psychiatric disorder.
Methods: A double-blind, placebo-controlled randomized study design was employed. The subjects received either 125 mg of SBME or placebo twice a day for a period of 12 weeks followed by a placebo period of another 4 weeks (total duration of the trial 16 weeks). Each subject was evaluated for cognition on a battery of tests comprising mental control, logical memory, digit forward, digit backward, visual reproduction and paired associate learning.
Results: SBME produced significant improvement on mental control, logical memory and paired associated learning during the 12-week drug therapy.
Conclusion: SBME is efficacious in subjects with age-associated memory impairment.
Source: Raghav et al. Indian Journal of Psychiatry, 2006; 48:238-242.
Significant improvement of performance on tests of working memory and visual information processing

Examining the Nootropic Effects of a special extract of Bacopa monniera on Human Cognitive Functioning
While Ayurvedic medicine has touted the cognitive enhancing effects of Bacopa monniera for centuries, there is a need for double-blind placebo-controlled investigations. One hundred and seven healthy participants were recruited for this double-blind placebo-controlled independent group design investigation. Sixty-two participants completed the study with 80% treatment compliance. Neuropsychological testing using the Cognitive Drug Research cognitive assessment system was conducted at baseline and after 90 days of treatment with a special extract of Bacopa monniera (2 × 150 mg KeenMind) or placebo. The Bacopa monniera product significantly improved performance on the ‘Working Memory’ factor, more specifically spatial working memory accuracy. The number of false-positives recorded in the Rapid visual information processing task was also reduced for the Bacopa monniera group following the treatment period. The current study provides support for the two other published studies reporting cognitive enhancing effects in healthy humans after a 90 day administration of the Bacopa monniera extract. Further studies are required to ascertain the effective dosage range, the time required to attain therapeutic levels and the effects over a longer term of administration.
Source:Stough et al.Phytotherapy Research, 2008; 22:1629-1634.
Improved performance for the CDB in participants consuming the 320 mg dose of Synapsa

An Acute, Double-Blind, Placebo-Controlled Crossover Study of 320 mg and 640 mg Doses of a Special Extract of Bacopa monnieri (CDRI 08) on Sustained Cognitive Performance
Abstract
Standardized extracts of the traditional Ayurvedic medicine Bacopa monnieri (BM) (Brahmi) have been recently shown to have cognitive enhancing effects in chronic administration studies. Pre-clinical work has also identified a number of acute anxiolytic, nootropic, and cardiovascular effects of BM. There has, however, been little research on the acute effects of BM on cognitive function. The current study aimed to assess the acute effects of a specific extract of BM (KeenMindW - CDRI 08) in a double-blind, placebo-controlled study in normal healthy participants who completed a cognitively demanding series of tests. Twenty-four healthy volunteers completed six repetitions of the Cognitive Demand Battery (CDB) after consuming a placebo, 320mg BM or 640mg of BM in a cross-over design and provided cardiovascular and mood assessments before and after treatment. Change frombaseline scores indicated that the 320mg dose ofBMimproved performance at the first, second, and fourth repetition post-dosing on the CDB, and the treatments had no effect upon cardiovascular activity or in attenuating task-induced ratings of stress and fatigue. It was concluded that assessment of an earlier pharmacological window and use of less memory-specific cognitive tests together with more temporally sensitivemeasures of brain activity may improve our understanding of the acute neurocognitive properties of BM.
Source:Downey et al. Phytotherapy Research 2012; 27:1407-1413.
Positive cognitive effects in measures of cognitive function, mood and cortisol levels

An Acute, Double-Blind, Placebo-Controlled Cross-over Study of 320 mg and 640 mg Doses of Bacopa monnieri (CDRI 08) on Multitasking Stress Reactivity and Mood.
Abstract
Little research exists in humans concerning the anxiolytic, antidepressant, sedative, and adaptogenic actions the traditional Ayurvedic medicine Bacopa monnieri (BM) possesses in addition to its documented cognitive-enhancing effects. Preclinical work has identified a number of acute anxiolytic, nootropic, and adaptogenic effects of BM that may also co-occur in humans. The current double-blind, placebo-controlled cross-over study assessed the acute effects of a specific extract of BM (KeenMind® - CDRI 08) in normal healthy participants during completion of a multitasking framework (MTF). Seventeen healthy volunteers completed the MTF, at baseline, then 1 h and 2 h after consuming a placebo, 320 mg BM and 640 mg of BM. Treatments were separated by a 7-day washout with order determined by Latin Square. Outcome measures included cognitive outcomes from the MTF, with mood and salivary cortisol measured before and after each completion of the MTF. Change from baseline scores indicated positive cognitive effects, notably at both 1 h post and 2 h post BM consumption on the Letter Search and Stroop tasks, suggesting an earlier nootropic effect of BM than previously investigated. There were also some positive mood effects and reduction in cortisol levels, pointing to a physiological mechanism for stress reduction associated with BM consumption. It was concluded that acute BM supplementation produced some adaptogenic and nootropic effects that need to be replicated in a larger sample and in isolation from stressful cognitive tests in order to quantify the magnitude of these effects.
Source:Benson S, Downey LA, Stough C et al. Phytotherapy Research 2013.
Improve attention, cognitive processing, and working memory via suppression of AChE activity

Effects of 12-Week Bacopa monnieri Consumption on Attention, Cognitive Processing, Working Memory, and Functions of Both Cholinergic and Monoaminergic Systems in Healthy Elderly Volunteers
Abstract
At present, the scientific evidence concerning the effect of Bacopa monnieri on brain activity together with working memory is less available. Therefore, we aimed to determine the effect of B. monnieri on attention, cognitive processing, working memory, and cholinergic and monoaminergic functions in healthy elderly. A randomized double-blind placebo-controlled design was utilized. Sixty healthy elderly subjects (mean age 62.62 years; SD 6.46), consisting of 23 males and 37 females, received either a standardized extract of B. monnieri (300 and 600 mg) or placebo once daily for 12 weeks. The cholinergic and monoaminergic systems functions were determined using AChE and MAO activities. Working memory was assessed using percent accuracy and reaction time of various memory tests as indices, whereas attention and cognitive processing were assessed using latencies and amplitude of N100 and P300 components of event-related potential. All assessments were performed before treatment, every four weeks throughout study period, and at four weeks after the cessation of intervention. B. monnieri-treated group showed improved working memory together with a decrease in both N100 and P300 latencies. The suppression of plasma AChE activity was also observed. These results suggest that B. monnieri can improve attention, cognitive processing, and working memory partly via the suppression of AChE activity.
Source:Evid Based Complement Alternat Med. 2012;2012:606424. doi: 10.1155/2012/606424. Epub 2012 Dec 18.
Safely enhancing cognitive performance in the aging

Effects of a Standardized Bacopa monnieri Extract on Cognitive Performance, Anxiety,and Depression in the Elderly: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Objectives: Study aims were to evaluate effects of Bacopa monnieri whole plant standardized dry extract on cognitive function and affect and its safety and tolerability in healthy elderly study participants.
Design: The study was a randomized, double-blind, placebo-controlled clinical trial with a placebo run-in of 6 weeks and a treatment period of 12 weeks.
Setting/location: Volunteers were recruited from the community to a clinic in Portland, Oregon by public notification.
Subjects: Fifty-four (54) participants, 65 or older (mean 73.5 years), without clinical signs of dementia, were recruited and randomized to Bacopa or placebo. Forty-eight (48) completed the study with 24 in each group.
Interventions: Standardized B. monnieri extract 300 mg/day or a similar placebo tablet orally for 12 weeks.
Outcome measures: The primary outcome variable was the delayed recall score from the Rey Auditory Verbal Learning Test (AVLT). Other cognitive measures were the Stroop Task assessing the ability to ignore irrelevant information, the Divided Attention Task (DAT), and the Wechsler Adult Intelligence Scale (WAIS) letter-digit test of immediate working memory. Affective measures were the State-Trait Anxiety Inventory, Center for Epidemiologic Studies Depression scale (CESD)-10 depression scale, and the Profile of Mood States. Vital signs were also monitored.
Results: Controlling for baseline cognitive deficit using the Blessed Orientation–Memory–Concentration test, Bacopa participants had enhanced AVLT delayed word recall memory scores relative to placebo. Stroop results were similarly significant, with the Bacopa group improving and the placebo group unchanged. CESD-10 depression scores, combined state plus trait anxiety scores, and heart rate decreased over time for the Bacopa group but increased for the placebo group. No effects were found on the DAT, WAIS digit task, mood, or blood pressure. The dose was well tolerated with few adverse events (Bacopa n=9, placebo n=10), primarily stomach upset.
Conclusions: This study provides further evidence that B. monnieri has potential for safely enhancing cognitive performance in the aging.
Source:The Journal of Alternative and Complementary Medicine Volume 14, Number 6, 2008, pp. 707–713
精选问答
Synapsa 是瑞尼琳牌记忆强化胶囊(CognitiPlus)的主要活性成分。 此款纯天然的,帮助增强记忆的 Synapsa 是一种独家拥有的,标准化的假马齿苋提取物,含有诸多的活性成分,有助于提高记忆力,学习能力和改善智力。 假马齿苋(也称为brahmi)是一种草药,几个世纪以来一直被传统的阿育吠陀医学用于维护大脑健康,改善人的认知功能。*
瑞尼琳牌记忆强化胶囊采用的Synapsa 活性成分是一种受专利保护的假马齿苋标准化提取物,具有30多年的临床研究历史,已被证明有助于在认知要求苛刻的环境中(如考试环境)帮助视觉处理,学习效率,工作记忆,信息保持和思维能力的改善。服用Synapsa 膳食补充剂有助于降低健忘率并提高多任务处理的准确性。*
Synapsa的研究已经在很多年龄段进行,从18岁到65岁甚至更年长的人群。 这意味着任何渴望提高学习和记忆能力的人都应该考虑这个选择,特别是那些想要在“多任务”环境中提高他们思维能力的人更应如此。*
瑞尼琳牌记忆强化胶囊采用的是标准化的 Synapsa 成分,它采用了一种受专利保护的提取工艺,遵循严格的种植和加工流程,以确保标准化的产品质量水平。该过程的每一个步骤都可能影响终产品的化学特性和治疗作用。 在每个步骤中实施种植和制造控制措施可以实现产品的一致性,从而产生出可供临床研究的产品。
根据临床验证的结果来看,90天期的临床研究一直采用 Synapsa 320毫克/天,而更高剂量(640毫克)已被用于评估Synapsa的急性或短期疗效。通常建议服用剂量为Synapsa 320毫克/天,也就是服用记忆强化胶囊每天一次,每次2粒。
对于一部分人来说,认知能力的提高产生的比较快。 研究表明服用 Synapsa 后2小时内思维能力就会有所提高。 关于针对记忆力和学习能力的长期研究结果已经显示,每日使用Synapsa,90天后记忆力和学习能力会得到改善。 继续每日使用 Synapsa 提供持续支持,以改善智能。*
瑞尼琳牌记忆强化胶囊采用的标准化的Synapsa 已在实验室和临床试验中研究了30多年。 在通常推荐的剂量研究中,已经证明它具有良好的耐受性,没有报道严重的副作用。 在极少数情况下,如果空腹服用可能会出现轻微的胃部不适。*
当然不一样。 Synapsa 采用专利工艺技术生产制造,它控制了从种子到植物提取的每一个环节。由于植物的栖息地,植物的使用部分,生长,收获和生产条件的不同,虽然同是假马齿苋提取物,质量上依然会有很大差异。
本网站提供的各项临床试验报告均以Synapsa 为对象进行。 你可能无法确定其他Bacopa monnieri 的成分在使用时的行为方式是否与 Synapsa 相同。
- * 本声明未经美国食品药品管理局评估。 本产品不用于诊断,治疗或预防任何疾病。 本网站提供的信息仅供您一般参考,不能代替专业医疗建议或针对特定疾病的治疗。